 |
|
| |  |
 |
 |
|
|
TIN PLATING
Introduction
Electrolytic tin is deposited in a direct current process on a cathodically connected product. An aqueous solution of tin salt and a tin anode are used. The use of organic additions to the baths enables decorative bright and functional deposits. Both acid and alkaline tin baths are employed.
Apart from pure tin, the metal is also applied as a component in alloy baths, such as in lead-tin plating.
Further information is available from the International Tin Research Institute www.surfacetec.co.uk
Properties
Tin can be deposited either dull or bright. A small amount of carbon and or sulphur is built in during deposition (< 0.1 wt %), the metal is very ductile, has good solderable and is very chemically resistant.
Bright tin is not very sensitive to staining by finger touch. It has good tribological and contact properties. It gives good protection to a steel substrate, the layer is not magnetic.
The resistance to adhesive wear is good. Layer hardness is between HV0,1 10 to 30 kg/mm2.
Electroplating process
Tin can be deposited on almost any metal:
- on steel parts for the food producing industry, and on cast steel mills for mincing meat
- on copper conductive components
- on copper alloys for increasing solderability
- on aluminium for light switches
- aluminium and copper alloys are generally precoated with another metal (‘strike’) in order to prevent diffusion between the substrate and tin.
Process characteristics
Electrolytic tin plating is carried out in:
- acid tin baths; sulfate and fluroborate tin baths (40 wt.%)
- alkaline tin baths; stannate baths (< 1 wt.%)
- methyl sulphonic acid baths; MSA baths (60 wt.%).
|
|
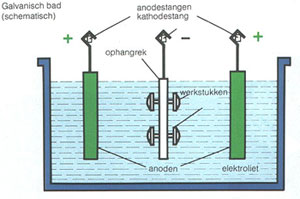
Schematics of an electrolytic bath.
Terms in Figure:
Galvanisch bad (schematisch) = Galvanic bath (schematics)
Anodestangen = Anode pole
Kathdesrang = cathode pole
Ophangrek = suspension grid
Werkstukken = products
|
The electroless deposition of tin is also possible (i.e. the deposition of tin using a chemical reaction, see chemical nickel) using a bath based on phenylsulphonic acid.
The deposition rate of acid baths is very high, the reason why they are frequently applied. The layers are uniform, but do not cover the substrate in holes etc.
Of all acid tin baths the fluoroborate bath is not often used. Acid tin baths are very suitable for drum plating and continuous plating. The process temperature is 20 to 25C.
A stannate bath is less sensitive for pollution, produces tin deposits which cover down holes, and for that reason is very suitable for profiling applications. The deposition rate is lower than that of acid baths. Process temperature between 60 and 80C. This process is being applied in only a few cases.
Methylsulphonic acid baths give very uniformly bright deposits. A wide range of applicable current densities (7 to 120 A/dm2 for continuous plating). This bath is often used to deposit lead-tin alloys.
Before tin plating the substrate should be cleaned following these steps:
- grit blasting
- degreasing by boiling in water
- rinsing
- electrolytic degreasing
- rinsing
- tarnishing/activation
- rinsing
Aluminium and copper alloys are often given a ‘strike’ layer to prevent diffusion.
Layer deposition
The parts to be tin plated are placed in an electrolyte and switched to the negative electrode (cathode). Smaller parts can be plated in drums (assuming they are not too fragile).
Current density in acid baths is 1 to 2 A/dm2, giving a deposition rate of 0.5 to 1 µm/min.
For a good corrosion protection on steel parts a layer thickness of minimum 50 µm is required. A thickness of 7.5 µm is needed to prevent discolouring of copper alloys in a moist environment.
5 to 7.5 µm is grown for increase of solderability. During continuous plating the deposition rate is up to 400 to 500 µm per hour (special process conditions).

|
|
Steel container with a tin layer of 5 to 7.5 µm. Application in electronics. The aim of the layer is to improve the solderability |
After treatment
Generally, no after-treatment is carried out, with the exception of thermal brightening (bright - melting) as is usual in the production of tinplate. Passivation is used for special applications (increase of chemical resistance of the tin).
For the prevention of hydrogen embrittlement parts may be heat treated at about 190C for several hours. Care should be taken in view of the low melting point of tin (232°C).
Advantages and disadvantages
Advantages
- Corrosion resistance and good resistance to adhesive wear
- Good solderability
- Resistance welding is possible after deposition of tin
- Not very sensitive to finger prints
- Good contact properties
- Approved for use in food industry ("FDA approved*").
[*FDA Approved: Food and Drug Administration Approved. FDA is an organisation which approves the use of a product (material or coating) in the food industry. Similar institutions are: USDA (United States Department of Agriculture) and NSF (National Sanitation Foundation)]
Disadvantages/Limitations
- High strength steel is sensitive to hydrogen embrittlement
- The tin layer completely covers the substrate, sometimes heat treatment is necessary in order to prevent brittleness
- Under certain conditions whisker* growth may appear from the tin layer
*Whisker growth: whiskers are microscopically small needles, in this case of tin oxides, which could cause short circuits in electrical contacts
 |
|
A whisker, a case of spontaneous crystal growth. Such whiskers could lead to short circuits in electronic parts.
|
Costs
Costs are dependent on the series, layer thickness and geometry of the parts. Also be considered is whether the parts can be plated in a drum or in an electrolytic bath.
Application
Tin layers are being used as decoration on jewellery and on antique tin objects.
The layers are generally applied for corrosion protection, protection against discolouring (e.g. as a result of oxidation at elevated temperatures, e.g. during soldering), and for optimising the solderability.
For functional applications tin plating is applied in the tin industry, food industry, electronics and telecommunication.
 |
|
Copper strips with a 15 µm thick tin layer, used for lowering the contact resistance. Application in railway switching boards |
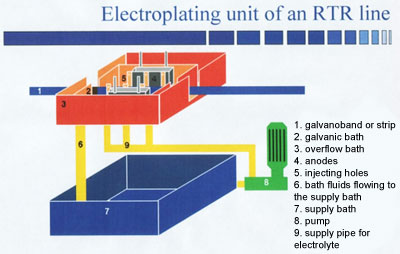
Schematic drawing of a reel-to-reel installation for the continuous deposition of tin on electronic parts with high rates.
| 
|
| 
|

|

|
|

